Answer:
The final volume is 18.38 L.
Step-by-step explanation:
It is given that,
A container is filled with 13.3 L of gas at 1.41 atm. The container is held at constant temperature throughout the experiment if the pressure changes to 1.02 atm.
It is based on Boyle's law. According to this law, at constant temperature,
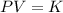
K is constant

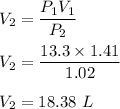
So, the final volume is 18.38 L.