Answer:
solubility of X in water at 17.0
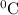 is 0.11 g/mL.
is 0.11 g/mL.
Step-by-step explanation:
Yes, the solubility of X in water at 17.0
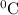 can be calculated using the information given.
can be calculated using the information given.
Let's assume solubility of X in water at 17.0
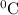 is y g/mL
is y g/mL
The geochemist ultimately got 3.96 g of crystals of X after evaporating the diluted solution made by diluting the 36.0 mL of stock solution.
So, solubility of X in 1 mL of water = y g
Hence, solubility of X in 36.0 mL of water = 36y g
So, 36y = 3.96
or, y =
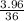 = 0.11
= 0.11
Hence solubility of X in water at 17.0
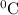 is 0.11 g/mL.
is 0.11 g/mL.