Answer:
The final volume is 1.6 L.
Step-by-step explanation:
It is given that,
A diver has a lung capacity of 2.4 L when the pressure is 0.8 atm. We need to find the volume of the diver’s lungs when the pressure changes to 1.2 atm. Let V₂ is volume.
It is based on Boyle's law. According to this law,
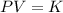
K is constant

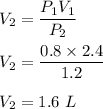
So, the final volume is 1.6 L.