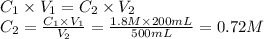Answer:
0.72 M
Step-by-step explanation:
Given data
- Initial volume of the H₂SO₄ solution (V₁): 200 mL
- Volume of water added (VH₂O): 300 mL
- Initial concentration of the H₂SO₄ solution (C₁): 1.8 M
Step 1: Calculate the final volume (V₂)
The final volume of the solution is equal to the sum of the initial volume of the solution and the volume of water.
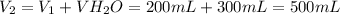
Step 2: Calculate the concentration of the diluted solution (C₂)
We will use the dilution rule.