Answer:
0.0198 mm/sec
Step-by-step explanation:
Given:
d = 8 cm
L = 10 m = 1000 cm
v = 200 m/min
E = 100%
density = 3500 A.m^-2
Using Faraday's law of electrolysis, we have:
Q = n(e) * F
Where,
Q = electric charge
F = Faraday's constant=96500Cmol
n(e) = electron charge = 2
Therefore
Q= 96500 * 2 = 193000
Let's find the area of pipe, A = πr²(L)
But radius, r =
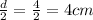
Therefore,
A = 3.142 * 4² * 1000
A = 50272
To find the thickness of the coating deposited, we have :
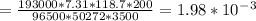
= 0.0198 mm/sec