Answer:
-112°C
Step-by-step explanation:
Given,
Pressure, P = 971 mmHg
moles of carbon dioxide = 3.3
Temperature, T = ?
Volume = 34.13 L
Pressure in atm =
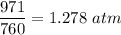
Now, using ideal gas equation
PV = n RT
1.278 x 34.13 =3.3 x 0.08206 x T
T = 161.07 K
Temperature in °C = 161.07-273 = -112°C
Temperature of sample is -112°C.