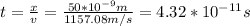Answer:
a) ∆x∆v = 5.78*10^-5
∆v = 1157.08 m/s
b) 4.32*10^{-11}
Step-by-step explanation:
To solve this problem you use the Heisenberg's uncertainty principle, that is given by:
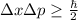
where h is the Planck's constant (6.62*10^-34 J s).
If you assume that the mass of the electron is constant you have:
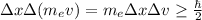
you use the value of the mass of an electron (9.61*10^-31 kg), and the uncertainty in the position of the electron (50nm), in order to calculate ∆x∆v and ∆v:
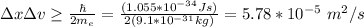
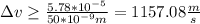
If the electron is a classical particle, the time it takes to traverse the channel is (by using the edge of the uncertainty in the velocity):