Answer:
200 liters.
Explanation:
To solve this, let's first set C in our equation to .7, since that is the concentration we are aiming for.
.7=
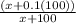
Next, we can simply this equation further:
.7=
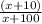
We can multiply both sides by the denominator, resulting in:
.7x+70= x+10
Finally, continue simplifying to solve for x:
60= .3x
x= 200 liters.