Answer:
B
Step-by-step explanation:
Since we want to find the volume of CO, but we're given Nickel's mass, we need to convert grams of Nickel to moles then convert that to moles of CO using stoichiometry from the balanced reaction.
The molar mass of Nickel is 58.69 g/mol:
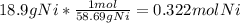
From the reaction, we can see that each mole of Nickel is 4 moles of CO, so to find the number of moles of CO from 0.322 moles of Nickel, multiply 0.322 by 4: 0.322 * 4 = 1.29 mol CO
Now, we can use the ideal gas law, PV = nRT, to find the volume of CO:
- the pressure P is 2.99 atm
- the volume is what we want to find
- the moles n is 1.29 mol CO
- the gas constant R here would be 0.08206 L atm / (mol K)
- the temperature T is 340.°C + 273 = 613 K
Plug all these values in:
PV = nRT
(2.99 atm) * V = (1.29 mol) * (0.08206) * (613 K)
V ≈ 21.7 L
The answer is B