Answer:
A
Step-by-step explanation:
First, we're given that the atmospheric pressure is 744.8 mmHg. However, this includes the pressure of the Zinc and the pressure of the water; we only want the pressure of the water, so subtract its pressures (23.8 mmHg) from 744.8 mmHg: 744.8 - 23.8 = 721 mmHg.
Now, we can use the ideal gas law: PV = nRT.
- the pressure P is 721 mmHg
- the volume V is 7.80 L
- the moles n of Zinc is what we want to find in order to calculate the mass
- the gas constant R is 62.36 L mmHg / (mol K)
- the temperature T is 25.0°C, or 25.0 + 273 = 298 K
Plug all these in:
PV = nRT
(721 mmHg) * (7.80 L) = n * (62.36) * (298 K)
n ≈ 0.303 mol Zn
We need to convert this to grams, so use the molar mass of Zinc, which is 65.38 g/mol:
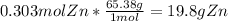
Thus, the answer is A.