Answer:
A. 4500 mL
Step-by-step explanation:
We need to use a version of the combined gas law, which states:
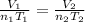 , where T represents temperature, V volume, and n moles
, where T represents temperature, V volume, and n moles
First, look at the initial values of n, V, and T:
- n: we have 10.0 grams of hydrogen, which we need to convert to moles. So:
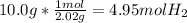
- V: we have a volume of 5.0 L
- T: our temperature right now is 28.0°C, but we need it in Kelvins, so add 273: 28.0 + 273 = 301 K
Now look at the final values:
- n: our final number of grams is 8.5 grams of hydrogen, so we need to convert this to moles.
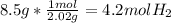
- V: this is what we want to find
- T: our final temperature is 44.0°C; to convert it to Kelvins, add 273.
44.0 + 273 = 317 K
Put this altogether:
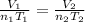
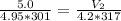
Multiply both sides by (4.2 * 317):
 ≈ 4.5 L
≈ 4.5 L
However, the problem wants millilitres, so simply multiply 4.5 by 1000:
4.5 * 1000 = 4500
The answer is thus A.