Answer:
a)exit velocity of the steam, V2 = 2016.8 ft/s
b) the amount of entropy produced is 0.006 Btu/Ibm.R
Step-by-step explanation:
Given:
P1 = 100 psi
V1 = 100 ft./sec
T1 = 500f
P2 = 40 psi
n = 95% = 0.95
a) for nozzle:
Let's apply steady gas equation.
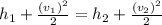
h1 and h2 = inlet and exit enthalpy respectively.
At T1 = 500f and P1 = 100 psi,
h1 = 1278.8 Btu/Ibm
s1 = 1.708 Btu/Ibm.R
At P2 = 40psi and s1 = 1.708 Btu/Ibm.R
1193.5 Btu/Ibm
Let's find the actual h2 using the formula :
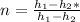
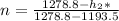
solving for h2, we have
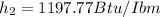
Take Btu/Ibm = 25037 ft²/s²
Using the first equation, exit velocity of the steam =
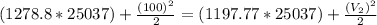
Solving for V2, we have
V2 = 2016.8 ft/s
b) The amount of entropy produced in BTU/ lbm R will be calculated using :
Δs = s2 - s1
Where s1 = 1.708 Btu/Ibm.R
At h2 = 1197.77 Btu/Ibm and P2 =40 psi,
S2 = 1.714 Btu/Ibm.R
Therefore, amount of entropy produced will be:
Δs = 1.714Btu/Ibm.R - 1.708Btu/Ibm.R
= 0.006 Btu/Ibm.R