Answer:
Theoretical yield is 20%.
Step-by-step explanation:
Percent yield = [(actual yield)/(theoretical yield)]
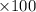 %
%
Theoretical yield is calculated amount of product by using stoichiometric ratio between reactant and product.
Actual yield is the amount of product which is experimentally obtained.
Here theoretical yield is 20.0 g and actual yield is 4.0 g.
So, percent yield =
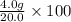 % = 20%
% = 20%