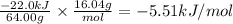Answer:
-5.51 kJ/mol
Step-by-step explanation:
Step 1: Calculate the heat required to heat the water.
We use the following expression.
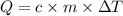
where,
- c: specific heat capacity
- m: mass
- ΔT: change in the temperature
The average density of water is 1 g/mL, so 75.0 mL ≅ 75.0 g.
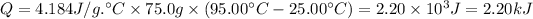
Step 2: Calculate the heat released by the methane
According to the law of conservation of energy, the sum of the heat released by the combustion of methane (Qc) and the heat absorbed by the water (Qw) is zero
Qc + Qw = 0
Qc = -Qw = -22.0 kJ
Step 3: Calculate the molar heat of combustion of methane.
The molar mass of methane is 16.04 g/mol. We use this data to find the molar heat of combustion of methane, considering that 22.0 kJ are released by the combustion of 64.00 g of methane.