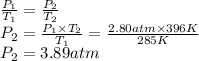Answer:
3.89 atm
Step-by-step explanation:
Given data
- Initial pressure (P₁): 2.80 atm
- Initial temperature (T₁): 285 K
- Initial volume (V₁): 15.6 L
- Final temperature (T₂): 396 K
- Final volume (V₂): 15.6 L (=V₁)
If we treat air as an ideal gas, we can calculate the final pressure using the Gay-Lussac's law.