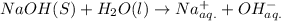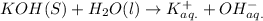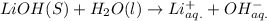Answer:
Arrhenius bases: NaOH, KOH and LiOH.
Step-by-step explanation:
Arrhenius bases are hydroxide (
 ) containing molecules which furnish
) containing molecules which furnish
 ion in aqueous solution.
ion in aqueous solution.
So, clearly, NaOH, KOH and LiOH are arrhenius bases as they contain
 ion as well as furnish
ion as well as furnish
 ion in aqueous solution.
ion in aqueous solution.
Hydrolysis in water: