Answer:
Heat, Q = 3035.073 J
Step-by-step explanation:
It is given that,
Mass of water, m = 15.5 g
Initial temperature,
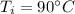
Final temperature,
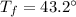
The specific heat of water is, c = 4.184 J/ g°C
The heat removed or absorbed by water is given by formula as :
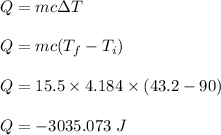
So, the heat of 3035.073 J is removed from 15.5 g of water.