Answer:
The current generated by the human per day = 89.34 Amperes
Step-by-step explanation:
Given that ;
the human consumes 20.0 mole of O₂ per day
The Food oxidation is given by the reaction :
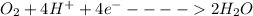
So; the human will produce 20.0 × 4 moles of e⁻
Thus; moles of e⁻ produced is = 80
Charge on 1 e⁻ = 1.602 × 10⁻¹⁹ C
e⁻ in 1 mole = 6.023 × 10²³
The total charge per day =
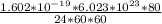
= 89.34 Amperes