Answer:
pH in the
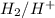 half cell is 0.84.
half cell is 0.84.
Step-by-step explanation:
Oxidation:
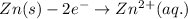
Reduction:
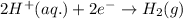
-------------------------------------------------------
Overall:
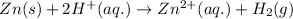
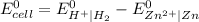 = (0.00 V) + (0.76 V) = 0.76 V
= (0.00 V) + (0.76 V) = 0.76 V
According to Nernst equation for this cell reaction at room temperature (298 K):
![E_(cell)=E_(cell)^(0)-(0.0592)/(n)log\frac{[Zn^(2+)].P_{H_(2)}}{[H^(+)]^(2)}](https://img.qammunity.org/2021/formulas/chemistry/college/5gx4qvvrohuc5otqyzjz93nf8qyo1isz39.png)
where,
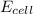 is cell potential, n is number of electron exchanged,
is cell potential, n is number of electron exchanged,
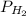 is pressure of
is pressure of
 in atm and species under third bracket represent molarity of the respective species.
in atm and species under third bracket represent molarity of the respective species.
So,
![0.68V=0.76V-(0.0592)/(2)log((1.3M)* (8atm))/([H^(+)]^(2))V](https://img.qammunity.org/2021/formulas/chemistry/college/gvva5jfxqyq4tllkirh42f709xq3xklx0z.png)

![[H^(+)]=0.1436M](https://img.qammunity.org/2021/formulas/chemistry/college/c1cvrbbtdwatvpl5jk0fxndwzkfj4slowd.png)
pH =
![-log[H^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/oj4yax9w9nnvk225iyqn6ycgsd2vftmzd8.png) = -log(0.1436) = 0.84
= -log(0.1436) = 0.84