Step-by-step explanation:
(a) The given data is as follows.
B =
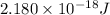
Z = 4 for Be
Now, for the first excited state
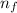 = 2; and
= 2; and
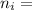 if it is ionized.
if it is ionized.
Therefore, ionization energy will be calculated as follows.
I.E =
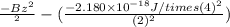
=
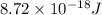
Converting this energy into kJ/mol as follows.
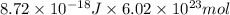
= 5249 kJ/mol
Therefore, the ionization energy of the
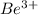 ion in its first excited state in kilojoules per mole is 5249 kJ/mol.
ion in its first excited state in kilojoules per mole is 5249 kJ/mol.
(b) Change in ionization energy is as follows.
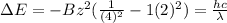
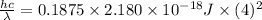
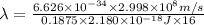
=
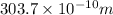
or, =
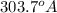
Therefore, wavelength of light given off from the
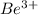 ion by electrons dropping from the fourth (n = 4) to the second (n = 2) energy levels
ion by electrons dropping from the fourth (n = 4) to the second (n = 2) energy levels
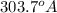 .
.