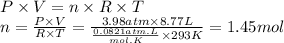Answer:
1.45 mol
Step-by-step explanation:
Given data
- Volume of the gas (V): 8.77 L
- Temperature of the gas (T): 20 °C
- Pressure of the gas (P): 3.98 atm
Step 1: Calculate the absolute temperature (Kelvin)
We will use the following expression.
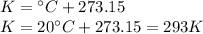
Step 2: Calculate the number of moles (n) of the gaseous sample
We will use the ideal gas equation.