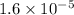Answer: The value of Keq for the reaction expressed in scientific notation is
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios.
For the given chemical reaction:
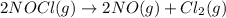
The expression for
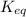 is written as:
is written as:
![K_(eq)=([NO]^2* [Cl_2])/([NOCl]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/651chsf7zxoqww9zzuyiujdx4q7xp8vr62.png)
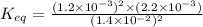
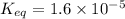
The value of Keq for the reaction expressed in scientific notation is