Answer:
= 14.24g of
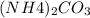 is required.
is required.
Step-by-step explanation:
Reaction equation:
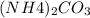 →
→
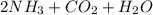
Mole ratio of ammonium carbonate to carbon dioxide is 1:1
1 mole of CO2 - 44g
?? mole of CO2 - 6.52g
= 6.52/44 = 0.148 moles was produced from this experiment.
Therefore, if 1 mole of
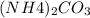 - 96.09 g
- 96.09 g
0.148 mol of
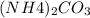 -- ?? g
-- ?? g
=0.148 × 96.09
= 14.24g of
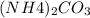 is required.
is required.