Answer:
the rate constant of the reaction at a temperature of 741 °C is
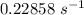
it will take 3.0313 s to consume half of the reactant if an identical experiment is performed at 741 °C
Step-by-step explanation:
Given that :
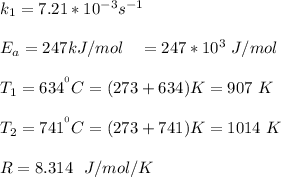
a)
According to Arrhenius Equation ;
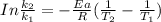
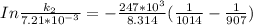
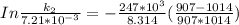
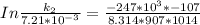
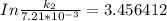
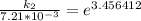
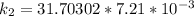
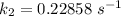
Therefore , the rate constant of the reaction at a temperature of 741 °C is
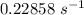
b) Given that :
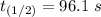
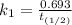
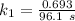
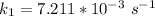
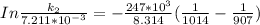
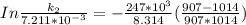
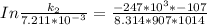
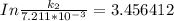
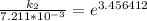
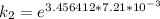
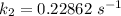
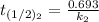
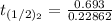
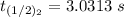
it will take 3.0313 s to consume half of the reactant if an identical experiment is performed at 741 °C