Answer: Volume of sample is 4.004 cm³
Explanation:
Given: Density of pure silver is 10.49 g/cm³
Mass of silver = 42 grams
To find: The volume of sample
As we know
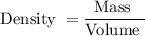
As given in question we have
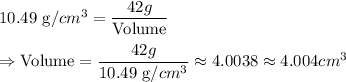
Hence 4. is the right option
Volume of sample is 4.004 cm³