Answer:
50 mL of 12M HCl should be added into 100 mL of water to produce a 4M solution.
Step-by-step explanation:
According to law of dilution-
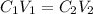
where
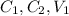 and
and
 are initial concentration, final concentration, initial volume and final volume respectively.
are initial concentration, final concentration, initial volume and final volume respectively.
Here,
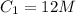 ,
,
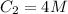 ,
,
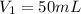
So,
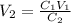 =
=
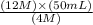 = 150 mL
= 150 mL
Hence, final volume of HCl solution should be 150 mL.
So, volume of water needed = (150-50) mL = 100 mL
Therefore 50 mL of 12M HCl should be added into 100 mL of water to produce a 4M solution.