Answer:
Check the explanation
Step-by-step explanation:
As we know the reaction of EDTA and
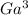 + and EDTA and
+ and EDTA and
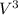 +
+
Let us say that the ratio is 1:1
Therefore, the number of moles of
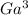 + = molarity * volume
+ = molarity * volume
= 0.0400M * 0.011L
= 0.00044 moles
Therefore excess EDTA moles = 0.00044 moles
Given , initial moles of EDTA = 0.0430 M * 0.025 L
= 0.001075
Therefore reacting moles of EDTA with
 = 0.001075 - 0.00044 = 0.000675 moles
= 0.001075 - 0.00044 = 0.000675 moles
Let us say that the ratio between
 and EDTA is 1:1
and EDTA is 1:1
Therefore moles of
 = 0.000675 moles
= 0.000675 moles
Molarity = moles / volume
= 0.000675 moles / 0.057 L
= 0.011 M (answer).