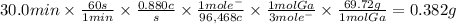Answer:
0.382 g
Step-by-step explanation:
Let's consider the reduction of gallium (III) to gallium that occurs in the electrolysis.
Ga³⁺ + 3 e⁻ → Ga
We can establish the following relations:
- 1 minute = 60 second
- 1 Ampere = 1 Coulomb / second
- The charge of 1 mole of electrons is 96,468 Coulomb (Faraday's constant)
- 1 mole of gallium is deposited when 3 moles of electrons circulate.
- The molar mass of gallium is 69.72 g/mol
We will use this that to determine the mass of gallium deposited from a Ga(III) solution using a current of 0.880 A that flows for 30.0 min