Answer: 7
Step-by-step explanation:
The volume of 0.18 M HClO4 is 100.0 mL.
Calculate the number of moles of 0.18 M HClO4 and 0.27 M LiOH as shown below:
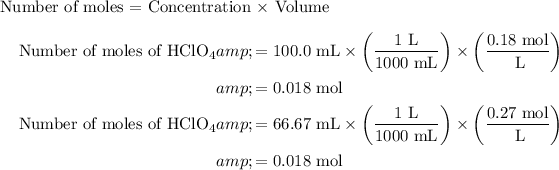
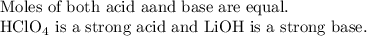
At the equivalence point, the number of moles of the acid completely reacted with all the moles of the added base. Thus, the solution becomes neutral in nature. The pH of a neutral solution is 7. Therefore, the pH of the solution at the equivalence point is 7.