Answer:
2.02*10^-9
Step-by-step explanation:
To find the fraction of the total you use the Boltzmann's distribution:
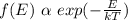 ( 1 )
( 1 )
E: energy of the particle = 10.2eV = 10.2 (1.6*10^-19 J) = 1.63*10^{-18} J
K: Boltzmann's constant = 1.38*10^{-23}J/K
T: temperature of the system = 5900K
That is, the probability to find a particle with energy E is inversely proportional to e^{E/KT}.
By replacing the values of the parameters in (1) you obtain:
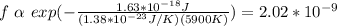
hence, approximately 2.02*10^-9 of the total hydrogen atoms are in the 2P states.