Answer:
0.036 moles of gas are contained in 890.0 mL at 21.0 C and 0.987 atm
Step-by-step explanation:
Ideal gases are those gases whose molecules do not interact with each other and move randomly.
An ideal gas is characterized by three state variables: absolute pressure (P), volume (V), and absolute temperature (T). The relationship between them constitutes the ideal gas law, an equation that relates the three variables if the amount of substance, number of moles n, remains constant and where R is the molar constant of the gases:
P * V = n * R * T
where P represents the pressure of the gas, V its volume, n the number of moles of gas (which must remain constant), R the constant of the gases and T the temperature of the gas.
In this case:
- P= 0.987 atm
- V= 890 mL= 0.890 L (being 1 L= 1,000 mL)
- n= ?
- R= 0.082
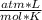
- T= 21 °C= 294 °K
Replacing:
0.987 atm* 0.890 L= n* 0.082
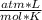 * 294 K
* 294 K
Solving:
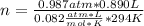
n= 0.036 moles
0.036 moles of gas are contained in 890.0 mL at 21.0 C and 0.987 atm