Answer:
(See explanation for further details).
Step-by-step explanation:
a) The real and ideal efficiencies are computed herein:
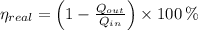
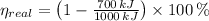
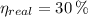
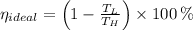
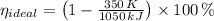
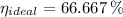
Since
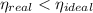 , the power cycle is irreversible.
, the power cycle is irreversible.
b) The differences between the real and ideal efficiencies is due to the existence of irreversibilities associated to the kind of the energy transformation processes and technological issues.