Answer:
200 grams of NaOH are produced with the reaction of 5.00 moles of water
Step-by-step explanation:
First of all you apply a rule of three to know the amount of moles of NaOH as follows: if 2 moles of water produce 2 moles of NaOH, 5 moles of water how many moles would they produce?
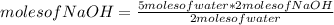
moles of NaOH= 5
Being:
- Na: 23 g/mole
- O: 16 g/mole
- H: 1 g/mole
the molar mass of NaOH is: 23 g/mole + 16 g/mole + 1 g/mole= 40 g/mole
Then a rule of three applies as follows: if in 1 mole there are 40 g of NaOH, in 5 moles how much mass is there?
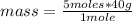
mass= 200 g
200 grams of NaOH are produced with the reaction of 5.00 moles of water