Answer:
The volume is decreasing at 160 cm³/min
Step-by-step explanation:
Given;
Boyle's law, PV = C
where;
P is pressure of the gas
V is volume of the gas
C is constant
Differentiate this equation using product rule:
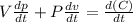
Given;
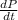 (increasing pressure rate of the gas) = 40 kPa/min
(increasing pressure rate of the gas) = 40 kPa/min
V (volume of the gas) = 600 cm³
P (pressure of the gas) = 150 kPa
Substitute in these values in the differential equation above and calculate the rate at which the volume is decreasing (
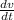 );
);
(600 x 40) + (150 x
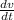 ) = 0
) = 0
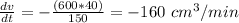
Therefore, the volume is decreasing at 160 cm³/min