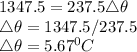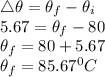Answer:
Final temperature of the hot coffee,
Step-by-step explanation:
Mass of coffee remaining after evaporation of 2.50 g, M = 240 - 2.50
M = 237.5 g
Mass of evaporated coffee, m = 2.5 g
Initial temperature of hot coffee,
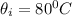
Initial temperature of hot coffee,
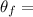 ?
?
Let the specific heat capacity of the coffee, c = 1 kcal/kg
Latent heat of vaporization of coffee,
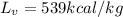
The heat energy due to temperature change:
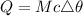
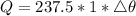 ...........(1)
...........(1)
The heat energy due to change in state
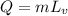
Q = 2.5 * 539
Q = 1347.5..........(2)
Equating (1) and (2)