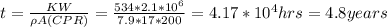Answer:
4.8 years
Step-by-step explanation:
The rate of corrosion (CPR) is defined as the rate at which a metal corrodes in a specific environment. It depends on the environmental condition and the type of metal. It is expressed in inches per year or melts per year. CPR is given by:
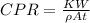
Where K is a constant = 534, W is the weight corroded = 2.1 kg = 2.1 × 10⁶mg, A is the area = 17 in², ρ is the density = 7.9 g/cm³
From the CPR equation: