Answer:
Step-by-step explanation:
Given that:
the temperature
 = 250 °C= ( 250+ 273.15 ) K = 523.15 K
= 250 °C= ( 250+ 273.15 ) K = 523.15 K
Pressure = 1800 kPa
a)
The truncated viral equation is expressed as:
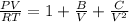
where; B = -
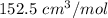 C = -5800
C = -5800
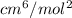
R = 8.314 × 10³ cm³ kPa. K⁻¹.mol⁻¹
Plugging all our values; we have
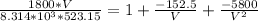
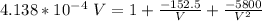
Multiplying through with V² ; we have
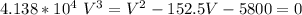
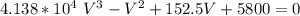
V = 2250.06 cm³ mol⁻¹
Z =
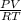
Z =
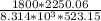
Z = 0.931
b) The truncated virial equation [Eq. (3.36)], with a value of B from the generalized Pitzer correlation [Eqs. (3.58)–(3.62)].
The generalized Pitzer correlation is :
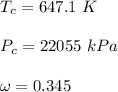
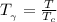
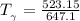
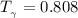
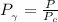
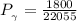
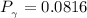
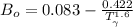
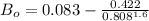

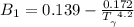
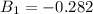
The compressibility is calculated as:
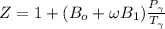
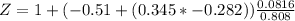
Z = 0.9386
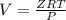
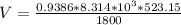
V = 2268.01 cm³ mol⁻¹
c) From the steam tables (App. E).
At
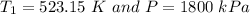
V = 0.1249 m³/ kg
M (molecular weight) = 18.015 gm/mol
V = 0.1249 × 10³ × 18.015
V = 2250.07 cm³/mol⁻¹
R = 729.77 J/kg.K
Z =
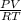
Z =
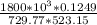
Z = 0.588