Answer:
d) 0.011%
Step-by-step explanation:
The probability for tunneling the barrier is given by the following formula:
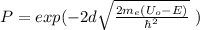 ( 1 )
( 1 )
me: mass of the electron
Uo: energy of the barrier
E: energy of the electron
d: thickness of the barrier
By replacing the values of the parameters in (1), you obtain:
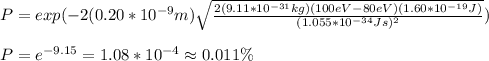
hence, the probability is 0.011% (answer d)