Answer:
The molarity of the diluted solution of NaOH is 0.225 M NaOH
Step-by-step explanation:
Here, we have, initial concentration of the solution = 0.90 M NaOH
Volume of initial solution = 125 mL
Volume of added water = 375 mL
Total volume of diluted solution = 375 mL + 125 mL = 500 mL
Number of moles in the diluted solution = 0.9 × 0.125 = 0.1125 moles of NaOH
Derived from the following relation;
0.9 M NaOH indicates that 1000 mL contains 0.9 moles of NaOH
Therefore, 1 mL contains 0.9/1000 moles of NaOH and 125 mL contains 0.9/1000×125 or 0.9 × 0.125 moles of NaOH
Therefore, the diluted 500 mL solution contains 0.1125 moles of NaOH. Therefore, 1000 mL of the diluted solution will contain
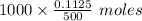 of NaOH or 0.225 moles of NaOH
of NaOH or 0.225 moles of NaOH
Hence the molarity of the diluted solution of NaOH, which is the number of moles per 1000 mL is 0.225 M.