Answer:
The energy required to melt 9.070 kg of ice is 3047520 J
Step-by-step explanation:
The mass, m of the ice = 9.070 kg = 907.0 g
The latent heat of fusion,
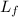 ° for ice = 3.36×10⁵ J/Kg
° for ice = 3.36×10⁵ J/Kg
Therefore we have the formula for the energy required to melt the mass m equals
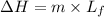
Plugging in the values, we have;
ΔH = 3.36 × 10⁵ J/Kg×9.070 kg = 3047520 J or
ΔH = 3.36 × 10² J/g×9070 g = 3047520 J
Therefore, the energy required to melt 9.070 kg of ice = 3047520 J.