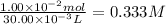Answer:
0.333 M
Step-by-step explanation:
Step 1: Write the balanced equation
2 HCl(aq) + Mg(OH)₂(aq) = MgCl₂(aq) + 2 H₂O(l)
Step 2: Calculate the reacting moles of Mg(OH)₂
25.00 mL of a 0.200 M magnesium hydroxide react. The reacting moles of Mg(OH)₂ are:
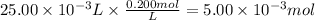
Step 3: Calculate the reacting moles of HCl
The molar ratio of HCl(aq) to Mg(OH)₂ is 2:1. The reacting moles of HCl are:
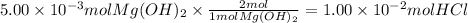
Step 4: Calculate the molarity of HCl