Answer:
The Celsius temperature of the gas is -259.34 ° C
Step-by-step explanation:
To answer the question, we note that the universal gas equation can be expressed as follows;
P·V = n·R·T
Therefore;
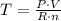
Where:
n = Number of moles = 2.49 mol
P = Pressure = 143 kPa = 143000 Pa
V = Volume = 2.00 L = 0.002 m³
T = Temperature in Kelvin, K = Required
R = Universal Gas Constant = 8.3145 J/(mol·K)
Plugging in the values, we have;
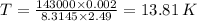
Hence the temperature of the gas = 13.81 K
Converting the Kelvin temperature to Celsius temperature, we have
Celsius temperature = Kelvin temperature - 273.15
Therefore, 13.81 K to Celsius temperature gives;
13.81 - 273.15 = -259.34 ° C.