Answer:
Approximately
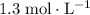 . (Assuming that the question says
. (Assuming that the question says
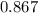 moles of salt in this
moles of salt in this
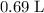 solution.)
solution.)
Step-by-step explanation:
The molarity of a solution gives the quantity of the solute in every unit volume of the solution. In this question:
- Quantity of solute:
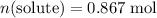 (with moles as the unit.)
(with moles as the unit.) - Volume of solution:
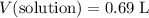 (with liters as the unit.)
(with liters as the unit.)
Note that in this question, liter is the unit for the volume of the solution. The molarity of the solution should thus give the amount of solute in every liter of the solution:
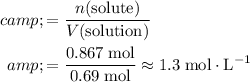 .
.