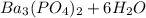Answer:
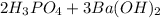 →
→
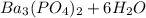
Step-by-step explanation:
Phosphoric acid is
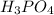 and barium hydroxide is
and barium hydroxide is
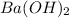 . Since this is an acid-base reaction, our products should be water and a salt. Put these given compounds together as the reactants:
. Since this is an acid-base reaction, our products should be water and a salt. Put these given compounds together as the reactants:
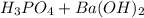 →
→
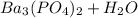
We need to balance this by making sure there are the same number of each atom on each side of the equation. Right now, on the left, we have:
- 5 H's
- 1 P
- 6 O's
- 1 Ba
On the right, we have:
- 2 H's
- 2 P's
- 5 O's
- 3 Ba's
To balance this, add a coefficient of 2 to the H3PO4, 3 to Ba(OH)2, and 6 to H2O:
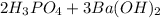 →
→