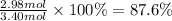Answer:
87.6 %
Step-by-step explanation:
Step 1: Write the balanced equation
2 AlF₃ + 3 Li₂CO₃ → Al₂(CO₃)₃ + 6 LiF
Step 2: Establish the appropriate molar ratio
The molar ratio of Li₂CO₃ to LiF is 3:6.
Step 3: Calculate the theoretical yield of LiF
We calculate how much LiF could be obtained from 1.70 moles of Li₂CO₃ if the percent yield were 100%.
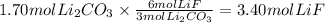
Step 4: Calculate the percent yield of the reaction