Answer: 12.5 grams.
Step-by-step explanation:
You can find the amount of pure substance remaining by using the half-life formula:
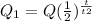
Where t is the amount of time, and t2 is the half life of the substance.
Substituting our values for the variables, we get this equation:
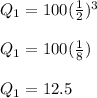
After 18 hours, there will be 12.5 grams of the substance remaining.