Answer:
After 18 hours, the amount of pure technetium that will be remaining is 12.5 grams
Step-by-step explanation:
To solve the question, we note that the equation for half life is as follows;
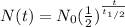
Where:
N(t) = Quantity of the remaining substance = Required quantity
N₀ = Initial radioactive substance quantity = 100 g
t = Time duration = 18 hours
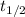 = Half life of the radioactive substance = 6 hours
= Half life of the radioactive substance = 6 hours
Therefore, plugging in the values, we have;
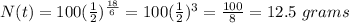
Therefore, after 18 hours, the amount of pure technetium that will be remaining = 12.5 grams.