Answer:
The molarity of the base is 0.2664 M
Step-by-step explanation:
Firstly, we write the complete and balanced titration reaction.
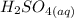 +
+
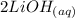 →
→
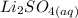 +
+
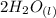
From the reaction, we can identify that 1 mole of the acid reacted with 2 moles of the alkali (base) to yield salt and water only
We identify the following also from the question;
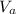 = 14.80 mL ,
= 14.80 mL ,
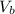 = 25.00 mL ,
= 25.00 mL ,
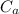 = 0.225M and
= 0.225M and
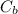 = ?
= ?
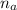 = 1 ,
= 1 ,
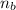 = 2
= 2
We use the relation;
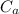
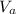 /
/
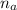 =
=
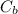
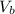 /
/
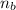
Plugging the values, we have ;
(0.225 × 14.80)/1 = (
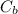 × 25.00)/2
× 25.00)/2
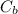 = (2 × 0.225 × 14.80)/25
= (2 × 0.225 × 14.80)/25
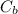 = 0.2664 M
= 0.2664 M