Answer:
a) E = 17.55 MeV
b) E = 18.99 MeV
c) E = 3.29 MeV
d) You can use the methods applied for the other parts to solve this, the equation is not properly written
e) E = 4.075 MeV
Step-by-step explanation:
Energy Released,
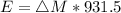
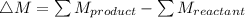
Mass of 1H,
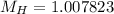
Mass of 2H,
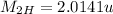
Mass of 3H,
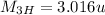
Mass of Helium,
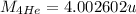
Mass of Beryllium,
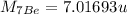
Mass of neutron,
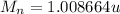
a)
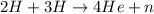
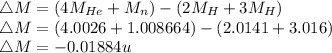
Energy released,
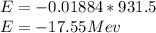
Energy released = 17.55 MeV
b)
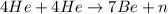
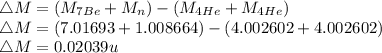
Energy released,
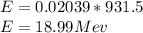
c)
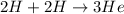 + n
+ n
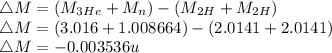
Energy released,
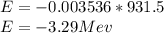
E = 3.29 MeV(Energy is released)
d) You can use the methods applied for the other parts to solve this, the equation is not properly written
e)
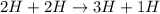
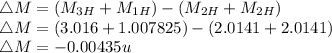
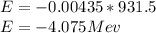
E = 4.075 MeV ( Energy is released)