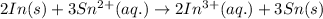Answer:
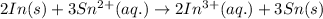
Step-by-step explanation:
In an oxidation reaction, electrons are being released from a species.
In a reduction reaction, electrons are being consumed by a species.
Here In release electrons and oxidized into
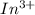 .
.
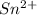 consume electrons and reduced to Sn.
consume electrons and reduced to Sn.
[Oxidation half-reaction:
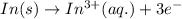 ]
]
[Reduction half-reaction:
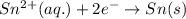 ]
]
-------------------------------------------------------------------------------
Overall reaction: